- Contact Person : Ms. Liu Vicky
- Company Name : Qinhuangdao Contec Medical Systems Co., Ltd.
- Tel : 86-335-8015418
- Fax : 86-335-8015590
- Address : Hebei,Qinhuangdao,No.112 Qinhuang West Avenue,Economic and Technology Development Zone
- Country/Region : China
- Zip : 066004
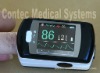
Finger Pulse Oximeter-FDA Approved
Major FeaturesIntegrated with SpO2 probe and processing display moduleSmall in volume,light in weight and convenient in carryingOperation of the product is simple ,low power consumptionSpO2 value displayPulse rate value display, bar graph displayLow-voltage indication: low-voltage indicator appears before working abnormally which is due to low-voltageAutomatically power off function: when the device is under the state of measuring interface . it will automatically power off within 5 seconds if the finger falls out of probe
Main performanceDisplay Mode: LED displaySpO2 Measuring Range: 0%~100%, (the resolution is 1%).Accuracy: 70%~100%: ±2% ,Below 70% unspecified.PR Measuring Range: 30bpm~250bpm, (the resolution is 1bpm)Accuracy: ±2bpm or ±2% (select larger)Measurement Performance in Weak Filling Condition:SpO2 and pulse rate can be shown correctly when pulse-filling ratio is 0.4%. SpO2 error is ±4%, pulse rate error is ±2 bpm or ±2% (select larger).Resistance to surrounding light: The deviation between the value measured in the condition of man-made light or indoor natural light and that of darkroom is less than ±1%.Power Consumption: less than 25 mAVoltage: DC 3.0VPower Supply: 1.5V (AAA size) alkaline batteries × 2 Battery working hour: The minimum continually work time is 24 hours, theoretical number is 56 hours.Safety Type: Interior Battery, BF Type
AccessoriesSell in standard:A hanging ropeA user manual
Wattanty
All items are brand new, with 3 years warranty and whole life repair
Qualification
FDA
FDA K Number
CMS50C Pulse OximeterPremarket Submission Number(510K): K073454Listing Number: D045684CMS50D/L/DL Pulse OximeterPremarket Submission Number(510K): K082641Listing Number: D064765Sonline A/B,Baby Sound A/B Pocket Fetal DopplerPremarket Submission Number(510K): K082480Listing Number: D072247
CMS50E,CMS50F,CMS60C,CMS60D Pulse OximeterPremarket Submission Number(510K): K090671 Listing Number:D078664, ECG80A ECG machinePremarket Submission Number(510K): K090936Listing Number: D081157
About UsContec Medical Systems is an original medical equipment manufactory based in China. We focus on researching, manufacturing and distributing of medical instruments. Welcome to us!
Finger Pulse Oximeter-FDA Approved